The medically minded optometrist looks for ways to expand his or her role when treating patients with age-related eye disease, glaucoma, postoperative pain, diabetic eye disease, and other conditions. It’s important that we practice to the extent of our licenses, which means writing prescriptions and, perhaps more important, knowing about the drugs both we and our MD counterparts are prescribing.
Keeping current with the most commonly prescribed ophthalmic drugs helps us be cognizant of and better able to identify adverse reactions or side effects our patients may be experiencing. It also allows us to explore other options when side effects surface or when one class of drug is contraindicated.
This article highlights some promising therapeutics in the pipeline for the treatment of ocular diseases, conditions, and symptoms.
AMD AND DIABETIC EYE DISEASE
Abicipar Pegol
Although the pathophysiology of diabetic retinopathy differs in many ways from that of age-related macular degeneration (AMD), treatment for diabetic macular edema and proliferative diabetic retinopathy includes suppression of the same stimulus that leads to retinal neovascularization: ischemia and subsequent upregulation of VEGF. Abicipar pegol (Allergan) is a designed ankyrin repeat protein, or DARPin, a therapeutic with high affinity for VEGF-A.1 This new anti-VEGF drug has shown efficacy similar or superior to that of ranibizumab (Lucentis, Genentech) injections in patients with wet AMD.2,3 Two identical global phase 3 studies (SEQUOIA and CEDAR) demonstrated the efficacy of a 12-week fixed dosing regimen of abicipar, with 50% fewer injections than ranibizumab, in the treatment of patients with neovascular AMD.4
GLAUCOMA
Omidenepag Isopropyl
A pivotal phase 3 US development program (SPECTRUM) investigating the use of omidenepag isopropyl 0.002% (DE-117, Santen) for the treatment of glaucoma or ocular hypertension (OHT) was initiated in the United States last year.5 This follows positive results from phase 1/2, 2, and 2b dosing studies demonstrating that 0.002% omidenepag is the most appropriate dose and that the investigational drug performed similarly to latanoprost in reducing IOP. Omidenepag, a selective agonist for the prostanoid receptor EP2, was found to be generally safe and well tolerated in the earlier studies. Common side effects of prostaglandin agonists (eg, iris and eyelid pigmentation, abnormal eyelash changes, deepening of upper eyelid sulcus) were not observed during long-term (12 months) use in a Japanese study.5
Microdose Latanoprost Formulation
A proprietary microdose formulation of latanoprost (MicroProst, Eyenovia) is being developed as a potential first-line treatment for the reduction of IOP in patients with chronic angle-closure glaucoma, OHT, or primary open-angle glaucoma. In a phase 2 feasibility dose-finding study, 30 healthy volunteers received single 8-µL microdoses of 0.005% lantanoprost (0.4 µg) using a high-precision, piezo-print horizontal delivery system on 2 successive days. This treatment reduced diurnal IOP from baseline at 1 and 2 days after administration. Patients successfully self-administered the microdoses after training, and administration was well tolerated and did not result in adverse events.6 The company expects to enroll the first patient in a phase 3 trial in the first half of this year.
Sustained-Release Bimatoprost Implant
Bimatoprost SR (Allergan) is an intracameral bimatoprost implant designed for sustained release. A first phase 3 study of the formulation, completed in mid 2018, showed good results with the device over a 12-week period, with comparable efficacy to daily use of a prostaglandin analogue and superior efficacy to daily timolol.7 When we discuss with patients the option of initiating medical treatment or performing selective laser trabeculoplasty in the setting of primary open angle glaucoma, an intracameral implantable device may be a viable alternative to topical medications. In patients who opt for laser treatment first, if a desired endpoint is not reached, this implant, if approved, may be a reasonable next step before initiating lifelong topical medical therapy.
POSTOPERATIVE PAIN AND INFLAMMATION
Loteprednol Gel
Submicron loteprednol etabonate ophthalmic gel 0.38% (Bausch + Lomb) is an investigational formulation that uses novel submicron particles to facilitate ocular penetration of loteprednol into key anterior segment tissues (eg, iris, ciliary body, aqueous humor, and cornea). If approved, this ophthalmic gel would be the lowest concentration loteprednol corticosteroid formulation indicated for the treatment of postoperative inflammation and pain after ocular surgery.
In September, it was reported that this investigational formulation of loteprednol met dual primary efficacy endpoints in a clinical trial: It was significantly more effective than vehicle in completely resolving ocular inflammation and pain after cataract surgery.8 Additionally, submicron loteprednol etabonate ophthalmic gel 0.38% had an acceptable safety profile regardless of whether it was administered two or three times per day.
DRY EYE
Reproxalap
In a recently completed phase 2b clinical trial, reproxalap topical ophthalmic solution (Aldeyra Therapeutics) improved both signs and symptoms of dry eye. Aldehydes are posited to play a role in potentiating ocular surface inflammation through reactive aldehyde species (RASP). In patients with dry eye disease, RASP may contribute to ocular inflammation. By diminishing aldehyde levels, Aldeyra’s topical ocular aldehyde trap platform is a novel approach that may augment existing therapy, and, in severe cases, reduce or eliminate the need for corticosteroids. Additional indications for reproxalap may include treatment of uveitis, chronic allergic conjunctivitis, and atopic ocular disease.9 A phase 3 study assessing the use of reproxalap 0.25% and 0.50% in treating allergic conjunctivitis was completed in November; results are pending publication.
SkQ1
SkQ1 (Visomitin, Mitotech) is a small molecule described by Mitotech as a cardiolipin peroxidation inhibitor, a compound designed to reduce oxidative stress within mitochondria. The company is exploring its use for several indications, including treatment of moderate to severe dry eye disease. In a phase 2 US clinical study of 90 patients at a single center, the topical ophthalmic formulation demonstrated statistically significant superiority over placebo for several endpoints, including fluorescein staining, ocular discomfort, and grittiness. SkQ1 was reported to be comfortable and well tolerated, and no unexpected or serious ocular adverse events occurred with its use. The first patient visit has been completed in a phase 3 multicenter clinical trial, VISTA-1, with three treatment arms (two concentrations of SkQ1 or placebo administered twice a day).10 Top-line results are expected to be released in the second quarter of 2019. SkQ1 has received marketing approval for dry eye disease in Russia.
ALLERGY
EM-100
In July, Eton Pharmaceuticals announced positive top-line results from a phase 3 study examining the efficacy of its preservative-free ophthalmic solution, EM-100, in the treatment of ocular itching.11,12 EM-100 demonstrated noninferiority to the over-the-counter comparator product, ketotifen fumarate ophthalmic solution 0.035% (Zaditor, Alcon), in relief of ocular itching and was also statistically significantly superior to placebo at all time points measured with no adverse events. The primary outcome measure in this study was ocular itching on a scale of 0 to 4 at various time points.
MYOPIA
Low-Dose Atropine
There are no FDA-approved therapies for slowing the progression of myopia, but in the pipeline with this goal in mind is the microdose therapeutic atropine (MicroPine, Eyenovia). The FDA recently accepted the company’s investigational new drug application to initiate the phase 3 CHAPERONE study, a US-based, multicenter, randomized, double-masked trial that will enroll more than 400 children between the ages of 5 and 12 years to test two concentrations of MicroPine and a placebo control arm in the treatment of progressive myopia.13
The OpteJet microdose formulation and delivery platform (Eyenovia) uses piezo-print technology to produce high-precision, volumetrically controlled topical medications to be applied directly to the ocular surface.
MYDRIASIS
Fixed Combination Microformulation
Phenylephrine/tropicamide (MicroStat, Eyenovia) is a fixed combination microformulation product candidate being developed for pharmacologic mydriasis. Two phase 3 trials of the drug have been completed, though no results have been posted to date.14,15 In the first trial, MIST-1, patients received either the fixed-combination phenylephrine 2.5%/tropicamide 1% ophthalmic solution or one of the two component drugs individually, all administed with the company’s OpteJet microdose dispenser.14 The second trial, MIST-2, compared the fixed combination with placebo.15 Data from both trials are expected in the first half of this year.
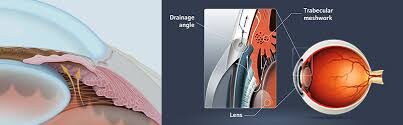
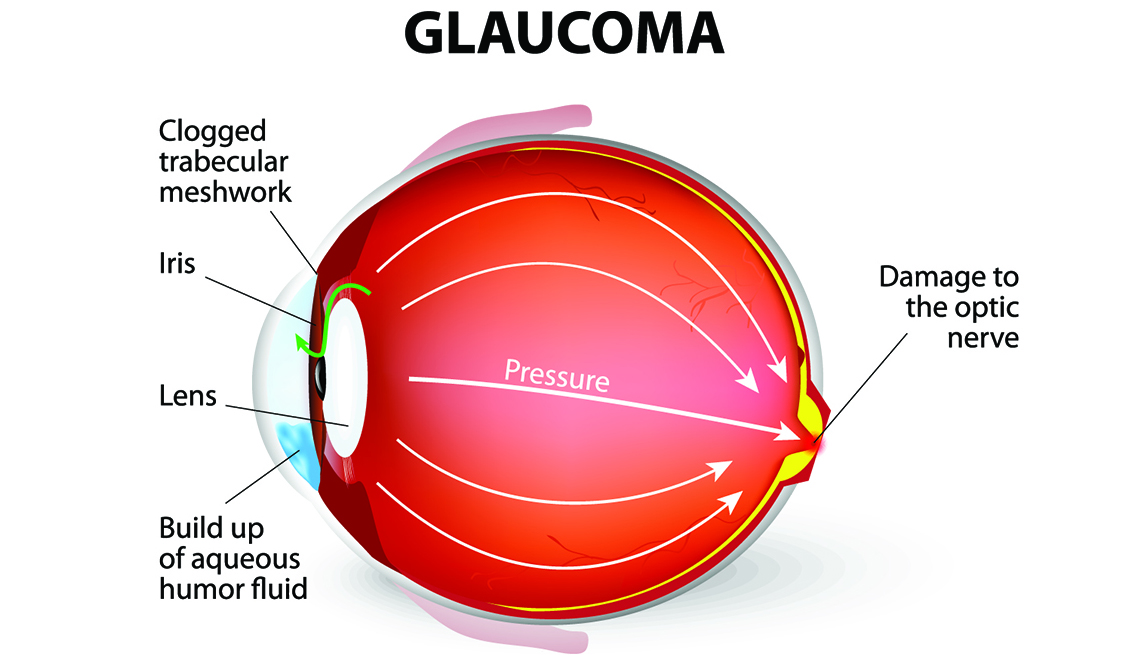
 Narrow angle and goniodysgenesis. These are the commonest forms of primary glaucoma in dogs. The drainage angle is abnormally formed. In narrow angle there is an abnormally narrow opening into the ciliary cleft. With goniodysgenesis the opening into the ciliary cleft is spanned by an abnormally differentiated pectinate ligament. The malformation of the drainage apparatus is heritable and the more severely affected animals will develop glaucoma. Other, poorly understood factors also play a role because although these abnormalities are present after ocular maturation, glaucoma does not develop until later in age (often middle age). There is a suggestion that further narrowing of the angle may occur with age. Screening dogs of at risk breeds can be performed to identify individuals with the predisposing anatomical abnormalities. Many breeds of dog are affected including Cocker spaniel, the Basset hound, arctic circle breeds (Samoyed, Siberian husky, Norwegian elkhound), Bouvier, terrier breeds, miniature poodle, Chow chow, Shar pei. This form of glaucoma typically has a very acute onset and results in very marked increases in IOP (above 60mmHg is common).
Narrow angle and goniodysgenesis. These are the commonest forms of primary glaucoma in dogs. The drainage angle is abnormally formed. In narrow angle there is an abnormally narrow opening into the ciliary cleft. With goniodysgenesis the opening into the ciliary cleft is spanned by an abnormally differentiated pectinate ligament. The malformation of the drainage apparatus is heritable and the more severely affected animals will develop glaucoma. Other, poorly understood factors also play a role because although these abnormalities are present after ocular maturation, glaucoma does not develop until later in age (often middle age). There is a suggestion that further narrowing of the angle may occur with age. Screening dogs of at risk breeds can be performed to identify individuals with the predisposing anatomical abnormalities. Many breeds of dog are affected including Cocker spaniel, the Basset hound, arctic circle breeds (Samoyed, Siberian husky, Norwegian elkhound), Bouvier, terrier breeds, miniature poodle, Chow chow, Shar pei. This form of glaucoma typically has a very acute onset and results in very marked increases in IOP (above 60mmHg is common). Severe pain (blepharospasm, photophobia, enophthalmos, elevation of the nictitating membrane, diffuse facial pain--trigeminal neuralgia--winces if head touched), slight epiphora. Altered behavior (hiding, off food). The signs of severe pain reduce a few days after onset.
Severe pain (blepharospasm, photophobia, enophthalmos, elevation of the nictitating membrane, diffuse facial pain--trigeminal neuralgia--winces if head touched), slight epiphora. Altered behavior (hiding, off food). The signs of severe pain reduce a few days after onset.